|
Summary Flow cytometry is an indispensable tool for diagnosis and monitoring of
leukemia and lymphoma. While application of flow cytometry in this field may be complex
and require a lot of experience, it is based on rather simple principles. With the help of
many figures this page is supposed to clearly explain these principles. |
|
|
|
|
|
|
|
|
|
|
|
I. Why
does the doctor request a flow cytometric analysis? Of course, there are a lot of indications for a flow cytometric analysis in
hematology. However, in principle, we can define 3 main indications.
- What's that?
The doctor discovers something, however, he needs further information about what
he sees?
- E.g., a patient shows an increase of lymphocytes in his blood. The
reason for this may be a leukemia or it may just be a reaction to a viral infection. With
flow cytometry this important discrimination is rather easy.
- Another example: in the microscope you definitely spot blasts, you
are sure that the patient has an acute leukemia. But what kind. It may be an acute
lymphocytic leukemia or an acute myeloid leukemia. This distinction is important for
therapy and for prognosis. Flow cytometry usually can give the answer.
- Sometimes flow cytometry helps to define the leukemia subgroup. For
example the so called M7, the megakaryoblastic leukemia.
Flow cytometry also helps to define lymphoma subgroups, for example the distinction
between CLL, hairy cell leukemia or other subgroups.
- Is there anything abnormal in the sample?
There is no particular suspicious cell population but the patient's symptoms suggest
leukemia or lymphoma as a possible cause.
Such cases are not easy and often unrewarding. Sometimes it is like searching for the
needle in the haystack. And sometimes we are even searching the wrong haystack, when blood
is sent to the lab but the lymphoma cells are only present in the bone marrow or in the
lymph node. The referring doctor should be aware of these limitations. And he should be
aware of what can and what can never be detected by flow cytometry in order to correctly
interpret a negative result.
- Is there still something?
When monitoring leukemia and lymphoma we try to find the malignant cells that were
detected originally.
That's an easy task when the malignant cells can be clearly separated from the normal
cells. In these cases even very small populations (e.g. 0.1% or less) of malignant
cells can be detected.
Monitoring is much more difficult when the malignant cells are very similar to normal
cells. Then, even larger population of malignant cells (e.g. 1 or 2%) may remain
undetected. In some cases of T-cell lymphoma even larger population may be unnoticed.
|
 |
|
|
|
|
II. How can we find an abnormal cell population? While in some cases the detection of abnormality may be very complex, the
underlying principles presented in the following figures are rather simple.
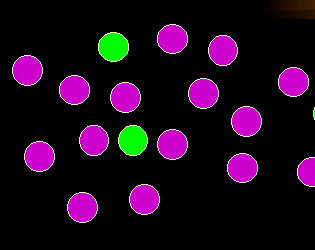 |
Normal finding
Let's assume these are the normal findings. A dominating population of green
"cells" and a few green cells. |
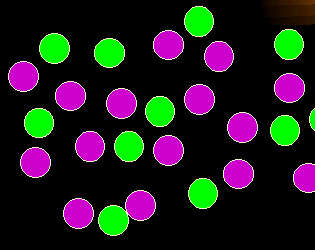 |
What's wrong in this picture?
You will notice immediately: too many green cells. In other words: a
disproportionate increase of one cell population.
As simple as this may sound, this kind of abnormality is an important sign for the
diagnosis of leukemia and lymphoma. |
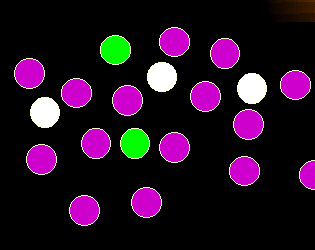 |
What's wrong in this picture?
There is a new cell population, the white cells. They were not present before. The
appearance of cells not normally present may be another another sign of leukemia
or lymphoma.
|
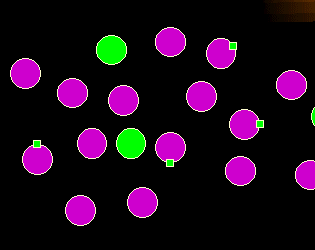 |
What's wrong here?
There is no disproportionate increase of any population, there are no cells that should
not be there but the magenta cells look different. They have something unusual on their
surface. The expression of abnormal antigens is another important sign
for malignancy.
|
 |
Finally, what's wrong here?
The bite-like deficiency in the magenta cells is supposed to symbolize the loss of an
antigen. Loss of an antigen normally present is another sign for
malignancy. |
That's it. As you can see, there are only a few principles to
detect abnormal cells. Application of these principles in many different ways helps to
distinguish normal from abnormal cells.
|
 |
|
|
|
|
III. Flow
cytometric analysis of mature B-cell neoplasms Preface
This chapter deals with malignant proliferations of mature B-cells (mature
B-Non-Hodgkin-Lymphoma, mature B-cell leukemia).
The distinction between lymphoma (disease present primarily in lymph nodes or
lymphatic organ/tissue) and leukemia (massive presence of malignant cells in blood or bone
marrow) is arbitrary.
Examples of such diseases are chronic lymphocytic leukemia (CLL),
hairy cell leukemia (HCL), prolymphocytic leukemia (PLL) as well as immunocytoma
(IC), mantle cell lymphoma (MCL), follicular lymphoma (FL) and diffuse large B-cell
lymphoma (DLBCL).
Why do we start with these diseases? Because flow cytometry plays a
big role for their diagnosis and classification.
Chapters:
|
 |
|
|
|
|
The
steps towards diagnosis of mature B-cell neoplasms
- Finding the abnormal B-cell population
(Are there abnormal, malignant B-cells at all?)
- Defining the
immunophenotype (the pattern of antigen expression) of the abnormal B-cell
population (which antigens do the malignant cells carry on their surface [and sometimes in
their cytoplasm]).
- Diagnosis
Sometimes flow cytometry can give the exact diagnosis, sometimes it helps to narrow down
the possible diagnoses.
Note: these steps are not necessarily analytical steps, they
are rather a logical concept to approach a diagnosis. |
 |
|
|
|
|
1. The search for monoclonal B-cells What are monoclonal B-cells?
A B-cell clone is a group of identical B-cells, originating from one cell, as is the case
in B-cell leukemia or lymphoma.
Why do we look for monoclonal B-cells?
Monoclonal B-cells are the single most important flow cytometric finding for the diagnosis
of a B-cell malignancy. If you do NOT find monoclonal B-cells (despite having looked
carefully and in the right place) a B-cell lymphoma or leukemia is very unlikely. Finding
monoclonal B-cells and corresponding symptoms in the patient makes a B-cell malignancy
very likely.
Are there monoclonal B-cell populations in the absence of
B-cell malignancy?
Yes, using methods as sensitive as flow cytometry, it is not uncommon to find small
monoclonal population in patients without any clinical sign of a B-cell malignancy. In
some cases this is supposedly a very early stage of a B-cell leukemia or lymphoma.
However, there is little data in the literature about the likelihood of progression of the
monoclonal population into a B-cell malignancy.
How can we find a monoclonal B-cell population using flow
cytometry?
With some cells it is very difficult to establish monoclonality, with B-cells, luckily, it
is relatively easy. The following figures should explain why:
|
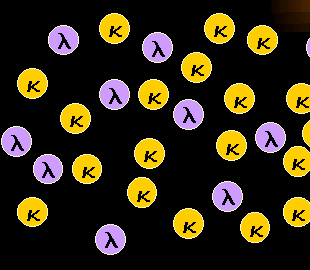
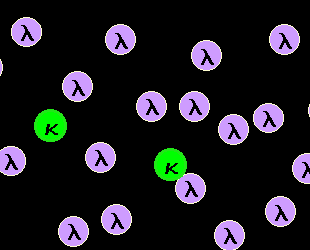
Normal, polyclonal B-cells are a mixture of
kappa-B-cells and lambda-B-cells.
Our B-cells have a special feature letting us detect their clonality easily: A B-cell
carries either kappa- or lambda-light chains on its surface. And normal polyclonal B-cells
are a mixture of kappa-B-cells and lambda B-cells as can be seen in the left-hand figure. |
|
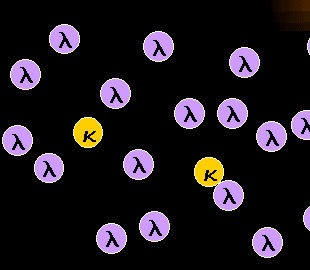
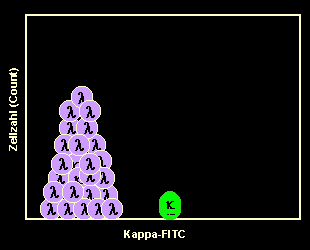
Monoclonal mature B-cells are either kappa or
lambda.
If a malignant B-cell clone proliferates this will result in a B-cell population
consisting of either only kappa- or only lambda-B-cells. The latter case (i.e.
lambda-monoclonal B-cells) is symbolized in the left-hand figure.
Note: in rare cases we find no light chain expressed on the B-cell surface, even in
a mature B-cell neoplasm. This makes the diagnosis a little bit more difficult. |
Thus we see that monoclonal B-cells are no mixture of kappa-
and lambda-B-cells like polyclonal B-cells, monoclonal B-cells show a gross preponderance
of either kappa- or lambda B-cells.
But how can we measure this by flow cytometry?
First, we stain the light chain (kappa or lambda) with suitable fluorescence-labeled
antibodies. Then we run the sample on the flow cytometer:
Assessment of B-cell
clonality on a flow cytometer
1. Normal, polyclonal B-cells |
|
Staining of kappa-B-cells
When we use an anti-kappa-FITC* antibody all kappa-B-cells will be stained.
Note: you may notice a slight preponderance of kappa cells. That is normal, one
usually has slightly more kappa- than lambda-B-cells.*FITC:
Fluoresceinisothiocyanate, a "green" fluorochrome. |
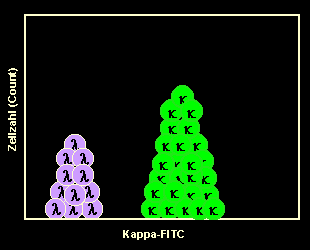
|
Running the sample on the flow cytometer
After measuring the sample on the cytometer we present the results in a so called
one-parameter histogram. You can see a slightly higher peak representing the
kappa-B-cells, which are stained by the antibody, and a smaller peak representing the
unstained lambda-B-cells. This is the typical picture of polyclonal B-cells. |
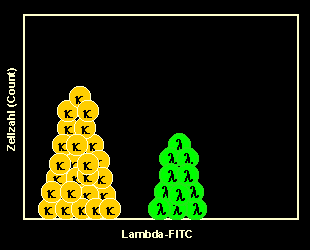
|
Staining of lambda-B-cells
In another tube we stain the lambda-B-cells using an anti-kappa-FITC antibody.
After measurement we encounter a somehow reversed image: A slightly smaller peak
representing the lambda-B-cells, which are stained by the antibody, and a larger peak
representing the unstained kappa-B-cells. Note:
in these examples we used a FITC-labeled antibody for kappa and lambda. Therefore,
staining had to be done in two separate tubes. Instead, one might use a
FITC(green)-labeled for kappa and a PE(orange)-labeled antibody for lambda. Then, one
might perform staining of both light chains in one tube. Both methods have their pros and
cons. In routine clinical practice we do both. |
In this first example the B-cell population was polyclonal,
suggested by the more or less even distribution of kappa- and lambda-B-cells
The next example looks different:
Assessment of B-cell
clonality on a flow cytometer
2. Abnormal, monoclonal B-cells |
|
Staining of kappa-B-cells
Only a few cells are stained because the majority of cells are lambda. |
|
Running the kappa-stained sample on the flow
cytometer
That's how the results of the flow cytometric measurement look like: There is
nearly no kappa-peak. The unstained B-cells dominate. |
|
Staining of lambda-B-cells
Accordingly, the stained B-cells dominate after staining with anti-lambda-FITC.
This suggests the presence of a monoclonal, lambda-positive (= a lambda-monoclonal) B-cell
population which may be leukemia or lymphoma cells. |
The figures shown so far were only schematic. In reality the
histograms generated after flow cytometric measurement look different. The following
figures show normal polyclonal as well as abnormal monoclonal B-cell populations:
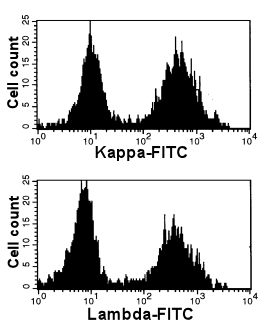 |
Normal, polyclonal B-cells
The two peaks after kappa- and lambda staining suggest an even distribution of kappa- and
lambda-B-cells. This suggests a normal polyclonal B-cell population.
Note (just in case you wondered): these histograms show that the majority
of B-cells is polyclonal. A very small monoclonal population, however, may remain
undetected. See below how to define clonality in a minor
B-cell population. |
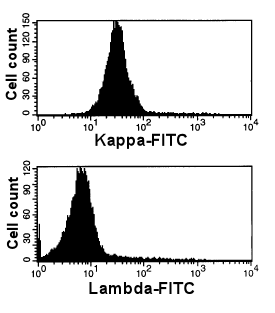 |
Abnormal, monoclonal B-cells
Upper panel: Here you see only one dominating peak. These cells are all
kappa-positive. This is due to the presence of a kappa-clonal B-cell clone. Nearly all of
the B-cells are part of the clone.
This is a case of chronic lymphatic leukemia (CLL).
Lower panel: The same cells are stained with anti-lambda-FITC. As expected, they
are negative. |
Without mentioning it, the figures above did only show the
B-cells, because these were our cells of interest. In a normal sample, however, we
encounter a number of different cell populations, of course. Not only B-cells. And these
cells may stain positive when staining with anti-kappa or anti-lambda antibodies and may,
therefore, obscure our results.
Nowadays this is not a big problem: in addition to a FITC-labeled anti-kappa or
anti-lambda we stain the cells with an anti-B-cell antibody, e.g. anti-CD19 or anti-CD20.
Then, we can display our results in a so-called two-parameter dot-plot, which may look
like this:
Normal, polyclonal
B-cells displayed in a 2-parameter dot-plot |
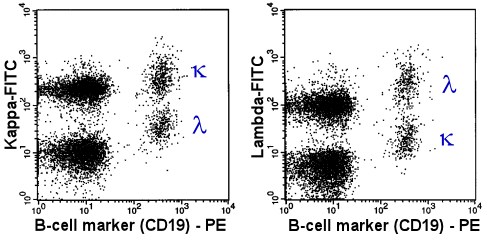 |
In 2 tubes the B-cells were stained with an
PE(orange)-labeled anti-CD19 antibody. Simultaneously, the light chain was stained with a
FITC-labeled antibody. Anti-kappa-FITC in the first tube (left panel) and anti-lambda-FITC
in the second tube (right panel). |
The population designated with the Greek k
and l are the kappa- and lambda-B-cells,
respectively. The distribution is more or less even. This suggests a polyclonal B-cell
population.
Note for those interested: the CD19-negative populations (on the left side of the
dot-plots) are T-cells (lower population) and monocytes (upper population). The monocytes
show a strong non specific binding of antibodies (via Fc-receptors) and therefore are
strongly positive when staining with anti-light chain or anti-heavy chain antibodies. |
The figure above shows the typical picture of a normal
polyclonal B-cell population.
Compare this picture to the light chain expression of the malignant cells in a case of
chronic lymphocytic Leukemia (below).
| Abnormal, monoclonal
B-cells displayed in a 2-parameter dot-plot |
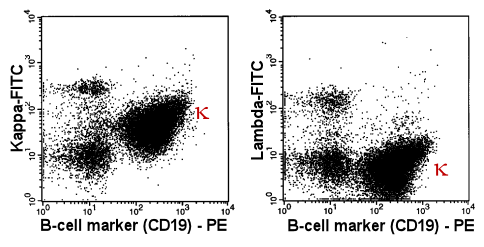 |
In 2 tubes the B-cells were stained with an PE-labeled
anti-CD19 antibody. Simultaneously, the light chain was stained with a FITC-labeled
antibody. Anti-kappa-FITC in the first tube (left panel) and anti-lambda-FITC in the
second tube (right panel). |
| The Greek k
designates the kappa-B-cells. Their extreme preponderance proves the monoclonality of the
B-cell population. |
Combining light chain staining with staining of other B-cell
antigens is not only done to identify the B-cells. It can also help to detect smaller
monoclonal B-cell populations.
This is shown in the next example:
Finding smaller monoclonal B-cell population by combined
measurement of various B-cell antigens |
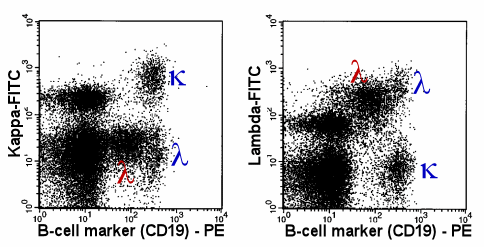 |
The lambda-monoclonal B-cells (red Greek l),
are easy to detect, because these cells show a weaker expression of CD19 than the normal,
polyclonal B-cells. |
| In this case the monoclonal population is rather
small. It is not dominating as in the previous case. Therefore, in a one-parameter
histogram it would be difficult to detect. In a 2-parameter dot-plot the monoclonal
population is easy to detect: its weaker CD19 expression separates the monoclonal cells
(red Greek l) from the normal polyclonal cells (blue Greek
k and l). |
It is not always CD19. Sometimes expression of CD20, CD79b,
CD5, CD10 or CD38 helps to discriminate normal from abnormal cells. By combining the
discriminating marker with kappa/lambda-staining, monoclonality can be established even in
very small B-cell populations.
|
 |
|
|
|
|
2. The search for B-cells with abnormal antigen expression Not as important as the search for monoclonal B-cells, but may help to find
abnormal B-cells.
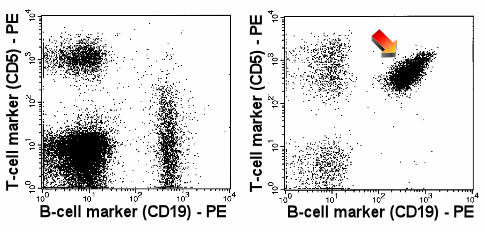 |
Expression of CD5
The arrow in the right panel points to the abnormal, strong expression of CD5 by B-cells.
CD5 expression as strong as this can usually only be found on T-cells. Normal B-cells show
no or only a weak expression of CD5 (left-hand panel) |
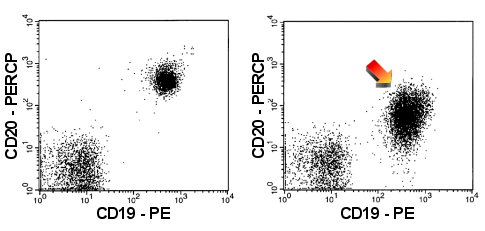 |
Weak expression of CD20
The B-cells in the right panel show only a weak expression of CD20 (arrow). For
comparison: normal CD20 expression in the left-hand panel. |
| In the right-hand dot-plots you find the B-cells
of a patient with CLL (Chronic Lymphocytic Leukemia), which typically show strong
expression of CD5 and weak expression of CD20. |
Caution: An abnormal antigen expression is
generally not a reliable sign of a B-cell malignancy. Especially when only a few B-cells
show the "abnormal" expression. In reactive conditions with B-cell activation or
even in healthy volunteers it is not uncommon to encounter small B-cell populations with
unusual antigen expression. If in doubt about its relevance always try to establish
clonality.
When detecting very small populations with abnormal or very unlikely antigen
expression one should also consider carry-over as possible reason. Many an unusual
population we have found during the last years has turned out just to be a carry over from
the previous tube. And some carry over can hardly be avoided, it is introduced by the
analyzers aspiration tube. |
 |
|
|
|
|
3. Defining the antigen expression
(immunophenotype) of the abnormal B-cells Once
you have established that there is a monoclonal, most likely malignant population you have
to define its immunophenotype, that means you have to check which antigens are expressed
on these cells an which are not. Usually that is an easy task. The way we do it, we have
stained all these antigens in the first place, so that now we just have to look at the
results.
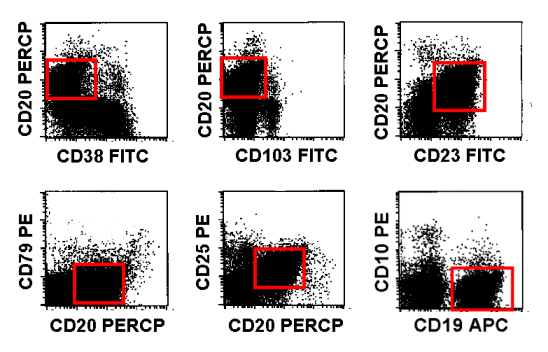 |
Immunophenotype of abnormal B-cells
The B-cells are CD38neg, CD103neg, CD23pos, CD79neg, CD25pos and CD10neg.PerCP: Peridinin Chlorophyll Protein
APC: Allophycocyanin |
|
 |
|
|
|
|
4. Diagnosis
of B-NHL Preliminary remark
This is a critical point. Can flow cytometry actually establish the diagnosis of a certain
B-NHL entity? Not always, strictly speaking not very often. However, flow cytometry can
often establish the presence of a clonal B-cell population when other techniques cannot
and flow cytometry results usually make certain lymphoma entities extremely likely and
others very unlikely. Therefore flow cytometry is an important, integral part of lymphoma
diagnosis even in cases where it cannot give a definitive diagnosis.
Procedure
In the last chapter we have defined the immunophenotype of the monoclonal B-cell
population. Now we have to find out which lymphoma entity might show such a phenotype.
To give an idea how that works, a table describing the typical immunophenotypes of the
most important mature B-cell neoplasms is shown below.
| |
CLL/SLL |
IC |
PLL |
MCL |
HCL |
FL |
surf.IG |
weakly + |
+ |
+ |
+ |
+ |
+ |
CD5 |
+ |
- to weakly + |
- to weakly + |
+ |
- |
- |
CD10 |
- |
- |
- |
- |
- |
+ |
CD11c |
- to + |
-/+ |
-/+ |
- |
strongly + |
- |
CD19 |
+ |
+ |
+ |
+ |
+ |
+ |
CD20 |
weakly + |
+ |
+ |
+ |
+ |
+ |
CD22 |
- to weakly + |
+ |
+/- |
+ |
+ |
+ |
CD23 |
+ |
- |
-/+ |
- |
- |
+/- |
CD24 |
+ |
+/- |
+ |
+ |
+ |
+ |
CD25 |
- to mod. + |
- to weakly + |
- |
- |
+ |
- |
CD38 |
- to mod. + |
+/- |
- |
|
rarely + |
|
CD43 |
+ |
+ |
+ |
+ |
- |
- |
CD79b |
- |
+ |
+ |
+ |
+ |
+ |
CD103 |
- |
- |
- |
- |
+ |
- |
FMC7 |
- |
+ |
+ |
+ |
+ |
+ |
CLL: Chronic Lymphocytic Leukemia
SLL: Small Lymphocytic Lymphoma
IC: Immunocytoma
PLL: Prolymphocytic Leukemia
MCL: Mantle Cell Lymphoma
HCL: Hairy Cell Leukemia
FL: Follicular Lymphoma |
It seems simple: you match the immunophenotype with that
described in the table and have the answer. Unfortunately this is not the case.
Primarily because of three problems:
- A certain immunophenotype may be typical but is by no means
obligatory
Example: a CLL is typically CD23pos and CD5pos. But it may be CD23neg and it may even be
CD5neg. And that goes for practically all antigens. Not one is present in all cases of a
certain B-NHL entity, hardly one is reliably never present in that entity.
Of course one important reason for this is that these entities are not defined by
immunophenotype but usually by morphology. That is why there are CD5-negative CLLs. In
fact, it is very likely that CD5-positive and CD5-negative CLL are different entities and
should probably not be lumped together in one group.
- The significance of one marker is depending on the expression
of the other markers
Example: If CD5 is positive, CD23 plays an important role because it helps discriminating
CLL from MCL. If CD5 is negative, CD23 expression is not really important.
- The strength of antigen expression is important
With some antigens it is not enough to describe it as positive or negative, it is
important how strongly the antigen is expressed. You rarely find this in tables
Mentioning these problems shall make clear that interpretation of
flow cytometric findings in hematological malignancy is more difficult than suggested by
tables like the one above or flow charts that can sometimes be found to guide flow
cytometric diagnosis.
|
 |
|
|
|
|
IV. Flow
cytometric analysis of mature T- or NK-cell neoplasms This chapter deals with malignant proliferations of mature T-cells
(T-non-Hodgkin-Lymphoma, T-NHL) and NK-cells. The distinction between lymphoma (disease
present primarily in lymph nodes or lymphatic organ/tissue) and leukemia (massive presence
of malignant cells in blood or bone marrow) is arbitrary.
Examples are the T-PLL (T-Prolymphocytic Leukemia), the T-LGL-
(T-Large-Granular-Lymphocyte-leukemia) and the NK-cell-leukemia.
The role of flow cytometry for the diagnosis of these diseases is
not as important as for B-NHL. Nevertheless, in many cases flow cytometry can find the
malignant T-cells or NK-cells and can guide the workup appropriately.
Chapters:
|
 |
|
|
|
|
The
steps towards diagnosis of mature malignant T- and NK-cell diseases
- Finding the abnormal T- or NK-cells
(Are there any malignant T- or NK-cells at all?)
- Search for T-cells with abnormal
antigen expression
In contrast to B-cells where the proof of monoclonality is pivotal, with T-cells finding
an abnormal antigen expression is more important. Why? Because finding an abnormal antigen
expression is usually all flow cytometry can do.
- Search for monoclonal
T-cells.
Unfortunately, it is rather difficult to establish a reliable, easy, routine flow
cytometric method for the detection of T-cell clonality. It requires a bundle of
antibodies and even then, clonality will not be reliably detected in all cases. With
NK-cells routine flow cytometry cannot prove monoclonality at all.
- Defining the immunophenotype
of the abnormal T- or NK-cell population. As soon as you have clearly defined and
discriminated the abnormal population this should not be very difficult.
- Finding the diagnosis
Sometimes flow cytometric results suggest which type of T-NHL is present, in other cases
flow cytometry can narrow down the possible entities.
Note: these steps are not necessarily analytical steps, they
are rather a logical concept to approach a diagnosis.
|
 |
|
|
|
|
1. Search for T-cells with abnormal antigen expression T-cells with an abnormal antigen expression may be a sign of a T-cell
malignancy. Abnormal antigen expression may be the loss of an antigen, expression of an
antigen not usually present or the over- or under-expression of an antigen.
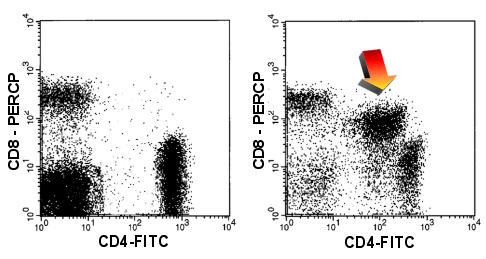 |
CD4/CD8
coexpression
In the right-hand dot-plot you can see cells that express both the CD4 and the CD8-antigen
(arrow) which is highly irregular. In addition both antigens are expressed weakly
(compared to normal T-cells). Left-hand panel shows a normal situation. |
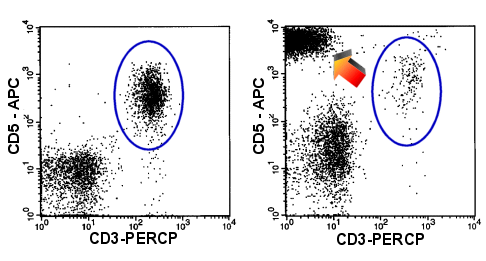 |
Loss of CD3
Overexpression of CD5
In the right-hand dot-plot you can see T-cells which overexpress CD5 while thy lack CD3
(arrow). Only a few normal T-cells are present. (blue oval). Left-hand panel shows a
normal situation. |
Caution: As mentioned for B-cells: an
abnormal antigen is generally not a reliable sign for T-cell malignancy. Especially if it
only concerns a few cells. Unusual T-cell populations may appear in many reactive
conditions. In B-NHL we can clear up a suspicious population by defining clonality. In
T-cells this usually is not possible. Therefore, in many cases we can only suspect a
T-cell lymphoma and suggest further lab tests. |
 |
|
|
|
|
2. Search for monoclonal T-cells Monoclonal T-cells are much more difficult to spot than monoclonal B-cells.
T-cells have nothing that compares to the kappa/lambda-ratio among B-cells.
The CD4/CD8-Ratio of T-cells (that is the ratio of CD4-expressing to CD8-expressing
T-cells) shows some similarity to the kappa/lambda ratio, however, there are certain
differences so that the CD4/CD8-ratio is by no way an equivalent tool to detect
monoclonality in T-cells. For a start, only extreme alterations of the CD4/CD8-Ratio may
be regarded as indicator of T-cell monoclonality. And only if this extraordinary high or
low ratio is caused by an increase of a T-cell population.
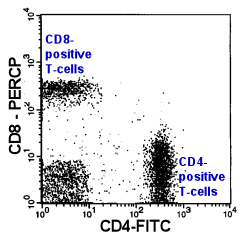 |
CD4/CD8-Ratio
Normally, the CD4/CD8-T-Cell ratio in peripheral blood is about 2:1. A In a T-lymphocytic
leukemia this ratio can shift dramatically. Unfortunately, this ratio may also be altered
by many non-malignant diseases. Therefore, only extreme alterations of this ratio can be
regarded as a sign for T-lymphocytic malignancy (and only if the ratio was altered by an
increase of one T-cell subgroup and not by a decrease of the other). |
Compared to the kappa/lambda-ratio the CD4/CD8-ratio
has many disadvantages: The CD4/CD8-Ratio can show strong variations also in non-malignant
disease, which means that only extreme alteration may be regarded as indicator of T-cell
monoclonality. But even this is only true if the abnormal ratio is caused by an increase
of one of the subpopulations. If it is due to a decrease this is no indicator of
monoclonality.
Another drawback is the following: If the kappa/lambda-ratio is equivocal (say a
ratio of 5, which may be due to a B-cell lymphoma or it may be not) one often succeeds in
isolating the potentially monoclonal B-cell population with some other markers and
determine the kappa/lambda-ratio of just this B-cell subpopulation. Then the
kappa/lambda-ratio may be 50, and monoclonality of the population has been proven. With
T-cells that is different. Of course you can isolate certain T-cell subpopulations and
check their CD4/CD8-ratios. But this will not help as many absolutely normal T-cell
subgroups consist only of CD4-positive T-cells others only of CD8-positive T-cells. An
extreme CD4/CD8-ratio among such a subpopulation is normal rather than a sign of
monoclonality.
|
 |
|
|
|
|
3. Defining
the antigen expression (immunophenotype) of the abnormal T/NK-cells Having defined the abnormal T-cells one can easily define the other antigens
expressed as described for B-cells.
|
 |
|
|
|
|
4. Diagnosis
of T-/NK-NHL-entity Preliminary remark
This is a critical point. Can flow cytometry actually establish the diagnosis of a certain
T-NHL entity? Not always, strictly speaking not very often. However, flow cytometry
results usually make certain lymphoma entities extremely likely and others very unlikely.
Therefore, flow cytometry is an important, integral part of lymphoma diagnosis even in
cases where it cannot give a definitive diagnosis.
Procedure
In the last chapter we have defined the immunophenotype of the abnormal T-cell population.
Now we have to find out which lymphoma entity might show such a phenotype.
To give an idea how that works, a table describing the typical immunophenotypes of the
most important mature T-cell neoplasms is shown below.
| |
T-PLL |
T-LGL |
NK-LGL |
Sezary-
Syndrome |
CD2 |
+ |
+ |
+ |
+ |
CD3 |
+ |
+ |
- |
+ |
CD4 |
+/- |
- |
- |
+ |
CD5 |
+ |
- |
+/- |
+ |
CD7 |
+ |
+/- |
+/- |
- |
CD8 |
-/+ |
+ |
+/- |
- |
CD16 |
- |
-/+ |
+/- |
- |
CD25 |
+/- |
- |
- |
- |
CD56 |
- |
- |
+/- |
- |
CD57 |
- |
+/- |
-/+ |
- |
HLA-DR |
-/+ |
- |
- |
- |
CD4+CD8+
coexpression |
25%
of cases |
|
|
|
T-PLL: T-Prolymphocytic
Leukemia
T-LGL: Large Granular Lymphocyte Leukemia (LGL), T-cell type
NK-LGL: LGL Leukemia, NK-cell type |
Seems simple but there are the same problems as described
above for B-cells:
- Certain phenotypes are typical but by no means obligatory.
For example: a Sezary syndrome is usually CD7 negative, but it may be positive. The same
is true for practically all other antigens.
- The significance of one marker depends on the presence of other
markers
- Not only the presence or absence of an antigen is important but also
the strength of its expression (i.e., the antigen density on the cells).
Mentioning these problems shall make clear that interpretation of
flow cytometric findings in hematological malignancy is more difficult than suggested by
tables like the one above or flow charts that can sometimes be found to guide flow
cytometric diagnosis.
|
 |
|
|
|
|
V. Flow
cytometric analysis of acute leukemia What
are acute luekemias?
Acute leukemias ("acute cancer of the blood") are caused by uncontrolled
proliferation of immature blood cells. The designation "acute" stems from the
fact that in contrast to chronic leukemia, acute leukemias would lead to death very
quickly without therapy.
How are cute leukemias categorized?
The most important categories are the acute myeloid leukemias (AMLs), which are caused by
immature myeloid cells, and the acute lymphocytic leukemias (ALLs), caused by lymphatic
cells. Further subgroups of these two entities can be defined.
Value of flow cytometric analysis
First, flow cytometry can help detecting an acute leukemia. However, usually this can be
done with microscopy as well and in some cases even better. Of greater value is flow
cytometry as a fast and reliable method to distinguish between AML and ALL, which is an
important decision. Moreover, flow cytometry can help defining the subtype to the acute
leukemia, primarily in cases of ALL, to a lesser extent also in cases of AML.
"Blasts"
The immature blood cells of an acute leukemia are generally called blasts. One has to be
aware, that in bone marrow a small number of blasts is absolutely normal.
Chapters:
|
 |
|
|
|
|
Steps
in the flow cytometric analysis of acute leukemia
- Finding the blast population
Are there blasts at all (in the peripheral blood) or are the blasts increased (in the bone
marrow)?
- Defining the immunophenotype
of the blast population
Which antigens do the blasts express (on their surface and in their cytoplasm)?
- Diagnosis
In virtually all cases flow cytometry can give the answer whether it is an AML or an ALL.
In some cases the subgroup can be defined, too.
Note: these steps are not necessarily analytical steps, they
are rather a logical concept to approach a diagnosis. |
 |
|
|
|
|
1. Finding
the blast population A malignant blast
population may be detected because of
- Increase of immature cells
- Abnormal marker expression of immature cells
a. Conspicuous increase of immature cells
There are many ways to detect immature cells by flow cytometry. One way is to
measure expression of antigens that are only present on immature cells, like CD34.
Unfortunately this antigen is not expressed on the immature cells in a considerable
proportion acute leukemia cases. A more reliable way to look for blasts is to inspect a
dot-plot showing CD45-Expression vs. Side Scatter. Immature cells are usually CD45 weaker
than lymphocytes and have a low side scatter signal. Therefore, they usually appear in
certain spots in the CD45/SSC-dot-plot. With this method one can detect blasts whether
they are CD34positive or not. In general one uses both, CD34 expression and the
CD45/SSC-dot plot.
Finding
immature cells |
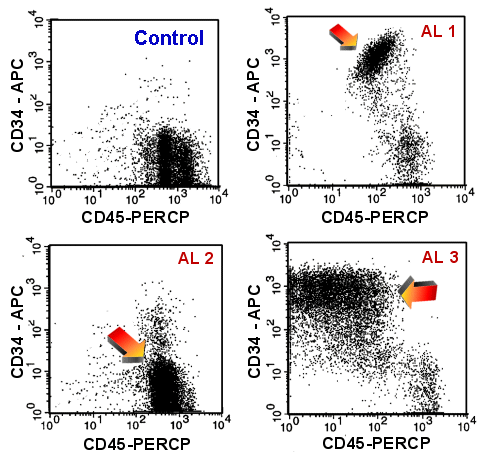 |
Finding immature cells using CD45-CD34
dot-plots
A healthy volunteer (upper left) and three cases of acute leukemia. The arrows points at
the blast populations, which is very conspicuous in case AL 1 (upper right) and
AL 3 (lower right).
In case AL 2 (lower left), the difference between between the normal picture
is more subtle and the blasts may be missed because in this case the blasts are CD34
negative. |
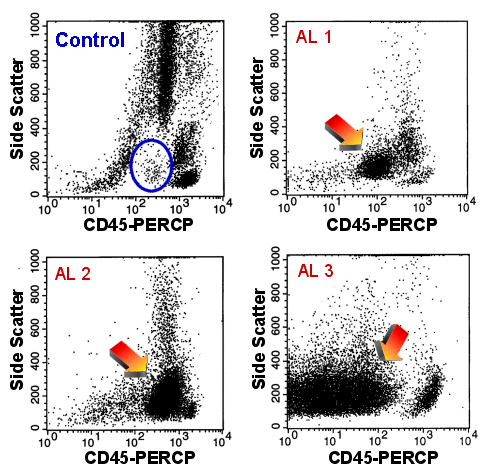 |
Finding immature cells using
CD45-Side Scatter dot-plots
The healthy volunteer (upper left): he shows only very few cells in the region surrounded
by the blue oval.
The three cases of acute leukemia: The arrows point to the blast populations which
are clearly visible in all three cases.
Even the blasts of case AL 2 can easily be spotted. |
Under normal conditions one finds only very few immature cells
in peripheral blood (less than 0.1 % of leukocytes) and even in bone marrow the
proportion of immature cells measured by flow cytometry remains relatively low (usually
less than 1 or 2 % of leukocytes). An acute leukemia is defined as showing more than
20 % blasts.
At this point it should be mentioned that if a cell
type has a high bone marrow / peripheral blood ratio (like blasts or plasma cells)
one usually underestimates its proportion in the bone marrow because the bone marrow
sample used for flow cytometry is diluted with peripheral blood. Therefore, it may
well happen that we find only 6 % blasts by flow cytometry while the pathologist
examining the bone marrow biopsy finds 25 %. Simply put: with flow cytometry we
examine a bone marrow/blood mixture, the pathologists examine "pure" bone
marrow.
b. Abnormal antigen expression of immature cells
There are different kinds of abnormal antigen expression.
The most important are:
- Expressing antigens of another cell lineage
It is abnormal to find lymphatic markers (like CD19, CD7) or markers like CD56 on
myeloid cells.
Inversely, it is also abnormal to find myeloid antigens like CD13 or CD33 on lymphatic
cells.
- Asynchronous expression of antigens
CD15 is usually only expressed on more mature neutrophils, finding it on CD34pos
blasts is abnormal.
- Abnormal intensity of antigen expression
In some cases of acute leukemia CD34 is expressed much more strongly than on normal
blast cells. T-ALLs often show a decreased expression of CD3, B-ALL a decreased or lacking
expression of CD20.
Most acute leukemias show a weak some a nearly lacking expression of CD45.
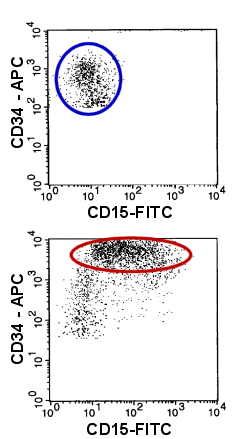 |
Example of an abnormal antigen expression on myeloid
blasts
Compare the normal blasts (upper dot-plot, blue oval) with those of an acute myeloid
leukemia (lower dot-plot, red oval)): the malignant blasts abnormally express CD15 and
they show an increased expression of CD34.
Note: CD34-negative cells have been removed for reason of clarity. |
|
 |
|
|
|
|
2. Defining
the immunophenotype of the blast population Once
the malignant blast population is discovered it is usually not very difficult to define
its phenotype as described for B-NHLs.
|
 |
|
|
|
|
3. Diagnosis a. Acute leukemia yes/no?
It may be possible to answer this question with flow cytometry, however, in many cases it
may be not. If we find more than 20% blast cells, the criteria of acute leukemia are met.
However, if we find less than 20% blast cells it may still be leukemia, as the number of
blasts in bone marrow may be considerably underestimated by flow cytometry as explained above. Very mature blast populations may pose another problem.
b. Myeloid or lymphoid blasts?
Flow cytometry can answer this question quickly and reliably. Some antigens are typical
for AML others for ALL. Some antigens are typical for B-ALL others for T-ALL.
How reliably a marker defines a lineage can be estimated from a table published by the
European group for the immunophenotyping of leukemias (EGIL).
This table was originally published to resolve the problem of acute leukemias
carrying the antigens of more than one lineage (biphenotypic leukemias).
EGIL-Score
for biphenotypic leukemias |
Score |
B-lymphocytic |
T -lymphocytic |
Myeloid |
2 |
CD79
(cyt/membrane)
CD22
(cyt/membrane)
cyt.IgM |
CD3
(cyt/membrane)
TCR-a/b
TCR-g/d |
Myelo-
peroxidase
(cytopl.) |
1 |
CD19
CD10
CD20 |
CD2
CD5
CD8
CD10 |
CD13
CD33
CDw65 |
0.5 |
TdT
CD24 |
|
CD14
CD15
CD64
CD117* |
The higher the score for an antigen the higher its value for
the lineage assignment. For example, myeloperoxidase (MPO) has the highest score for the
definition of AML because it is never present in ALL, CD79 is very important for the
diagnosis of B-ALL because it virtually never is expressed in AML or T-ALL.
*Of note, since the original publication of this table CD117 has turned out to be
more valuable for detecting myeloid lineage than initially thought. At least one score
point for myeloid should be allocated if CD117 is expressed on the blast cells.
c. Definition of the acute leukemia subgroup
AML and ALL are divided in certain subgroups.
- Flow cytometry can elegantly define the subgroups of B-ALL
(Pro-B-ALL, common B-ALL, pre-B-ALL and B-ALL) because these groups have been defined
according to antigen expression.
- Flow cytometric categorization of T-ALL is slightly less relevant
than that of B-cells.
- With AML, flow cytometry can help to detect special subgroups. It is,
for example, rather difficult to prove an AML-M7 (megakaryoblastic leukemia) without flow
cytometry. Flow cytometry can detect certain antigens (CD41, CD61) on the blast cells
which are typical for AML-M7.
BTW, this sounds easier than it is. This is a very tricky task. One is easily
fooled by platelets stuck to the blast cells.
Flow cytometry can also help diagnose other AML-subgroups, but the
emphasis is on the word 'help'. These groups are defined by their morphology,
cytochemistry or cytogenetics and can, therefore, not be reliably defined by flow
cytometry.
|
 |




